The mini gut is incubated with the food toxin deoxynivalenol (DON), now sadly global in its reach, to actively damage and perforate the gut, resulting in what we call a “leaky gut”. This leaky gut can then be test-treated with substances that have a potentially protective or healing effect in a physiologically much more realistic setting than available in other, common in vitro models used to date. With a markedly high throughput level for 3D systems, this practical model of the gut can also help to reduce the number of animal experiments. The DON-based leaky gut model allows the effect of potentially protective substances or other influential factors on gut integrity to be tested.
Gut-on-a-chip
Let it flow!
3D-Darmröhre
A 3D model of the gut on a microfluidic chip has been established in-house in order to explore the effect of protective substances on the intestinal barrier. The model meets the growing physiological requirements for in vitro models. Using this system the intestinal cells not only grow to form 3D “mini guts”, they also encourage substances to flow through them, mirroring the process of peristalsis in our guts.
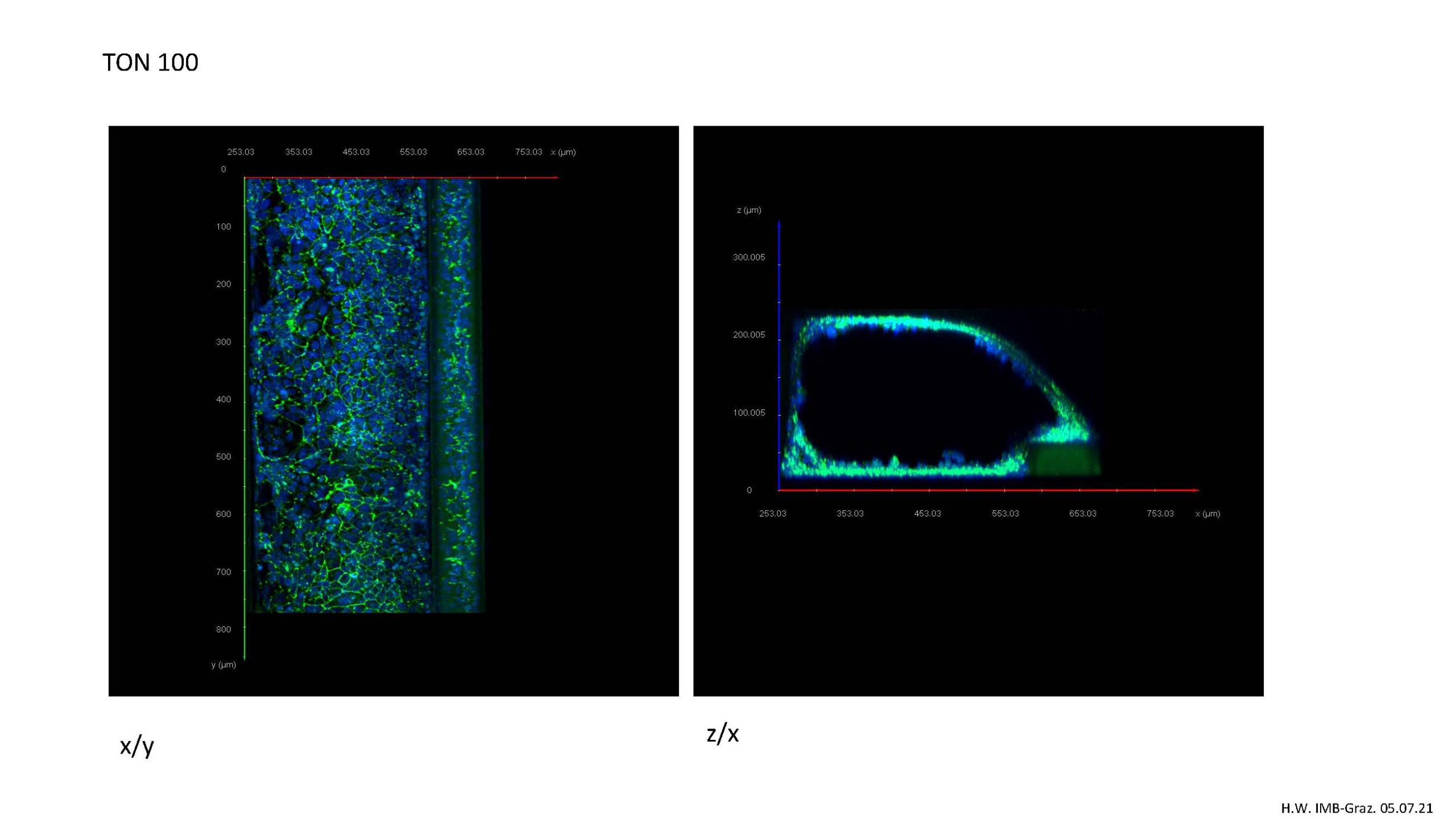
The mini gut on the chip: Longitudinal (left) and cross-sectional (right) image of a 3D mini gut stained with immunofluorescence dye (anti-zonulin 1), prepared by Dr Heimo Wolinski, IMB, University of Graz

The mycotoxin deoxynivalenol (DON) is prevalent worldwide and is a contaminant in many grain-based foods. Where consumption is excessive (vegan diet, young children (!)), this can lead to a variety of pathological symptoms. Acute symptoms of poisoning include diarrhoea, nausea and vomiting, while the long-term, chronic ingestion of DON can lead to outcomes including greater susceptibility to infection and growth disorders. Animal experiments and in vitro studies with conventional 2D cell cultures have already shown that the toxin causes damage to the intestinal barrier which, in turn, can trigger the symptoms outlined above.
However, physiological conditions that are as realistic as possible and do not involve animal experiments should be selected for more closely characterizing the effect of DON on the intestinal barrier and testing possible protective substances. That was the reason for choosing a Mimetas organ-on-a-chip model on which to create a 3D mini gut through which substances can flow in vitro. The project succeeded in establishing and characterizing the DON-based leaky gut model and is ready for use in testing potential protective substances and mycotoxin mixtures.
References / Publications
- Pöschl F, Höher T, Pirklbauer S, Wolinski H, Lienhart L, Ressler M, Riederer M., Dose and route dependent effects of the mycotoxin deoxynivalenol in a 3D gut-on-a-chip model with flow, Toxicol In Vitro, 2023 Apr; 88:105563. doi: 10.1016/j.tiv.2023.105563. Epub 2023 Jan 26. PMID: 36709839.
- C. Martins, D. Torres, C. Lopes, D. Correia, A. Goios, R. Assunção, et al., Deoxynivalenol exposure assessment through a modelling approach of food intake and biomonitoring data – a contribution to the risk assessment of an enteropathogenic mycotoxin, Food Res. Int., 140 (2021), Article 109863
- European Food Safety Authority, Deoxynivalenol in Food and Feed: Occurrence and Exposure, EFS2 (2013), p. 11, 10.2903/j.efsa.2013.3379
- Y. Gao, L. Meng, H. Liu, J. Wang, N. Zheng, The compromised intestinal barrier induced by mycotoxins, Toxins, 12 (2020), p. 619